Genetic forms of hearing loss and deafness can be syndromic (associated with malformation of the external ear or other external organs with medical problems involving other organ systems), or non-syndromic (no associated visible abnormalities of the external ear or related medical problems). Among the genetic forms, approximately 30% are syndromic, while 70% are non-syndromic. So far, more than 400 genes have been identified which are responsible for hearing impairment.
Syndromic Hearing Impairment
Patterns of Inheritance
Hearing deficits can be inherited in one of the four ways:
- 1. Autosomal dominant
- 2. Autosomal recessive
- 3. X-link
- 4. Mitochondrial patterns of inheritance
In an autosomal dominant pattern of inheritance (Fig.1), a child inherits a normal copy of a gene from one parent and an abnormal gene from the other parent. The abnormal gene dominates the normal gene, so one copy of an abnormal gene is sufficient to cause an autosomal dominant disorder. Waardenburg syndrome is the most common cause of autosomal dominant syndromic hearing loss, affecting 1 in 42,000 people. Other hearing impairments inherited in this fashion include Branchiootorenal syndrome, Stickler syndrome and Neurofibromatosis 2.
Autosomal Recessive: In this pattern of inheritance, two copies of the abnormal gene are required to cause the disorder (Fig-2). Individuals who inherit only one abnormal gene and one normal gene are referred to as carriers and are not affected by the disorder. Individuals affected by an autosomal recessive disorder usually are the result of mating between two carriers. Consanguinity increases the risk of an autosomal recessive disorder.Each child of two carrier parents has a 25% chance of inheriting two abnormal copies and thus having hearing loss. However, autosomal recessive disorders are typically not seen in every generation of an affected family because an abnormal copy of the gene may be passed on from one (unaffected) carrier to the next for many generations before a couple, who by chance both carry an abnormal copy, has an affected child. Usher syndrome is the most common type of autosomal recessive syndromic hearing loss. Others include Pendred syndrome and Jervell & Lange-Nielsen syndrome.
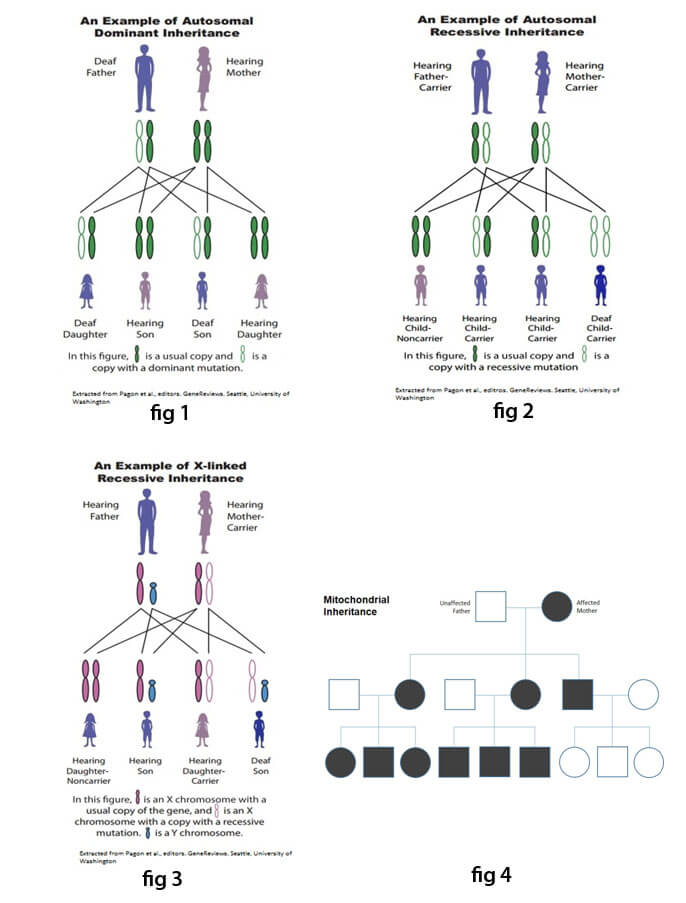
X-linked hearing defects are inherited through genes on the X chromosome (Fig.3). Males only carry one X chromosome. Therefore, they are more susceptible to an X-linked disorder. In X-linked disorders, affected fathers usually pass on the abnormal gene to their daughters, but not to their sons. More than 80% of Alport syndrome patients have an X-linked disorder associated with sensorineural hearing loss.
Mitochondrial patterns of inheritance: Mitochondrial disorders are inherited exclusively through the maternal line. Children inherit all of the mtDNA from mothers, so if the hearing loss is caused by a mutation in mtDNA, all the children of affected mothers (but none of the children of affected fathers) would be expected to have hearing loss. A mtDNA mutation, in which a G instead of an A is found at position 1555 (called A1555G), causes severe to profound sensorineural hearing loss.
Candidate Genes for Syndromic Hearing Impairment
Autosomal dominant syndromic hearing impairment| Disorder | Subtype | Gene |
|---|---|---|
| Waardenburg syndrome | WS I | PAX3 |
| WS II | MITF | |
| WS III | PAX3 | |
| WS IV | EDNRB,EDN3,SOX10 | |
| Branchiootorenal syndrome | EYA 1, SIX1,SIX5 | |
| Stickeler Syndrome | STL1 | COL2A1 |
| STL2 | COL11A1 | |
| STL3 | COL11A2 | |
| Neurofibromastosis2 | NF2 |
Autosomal recessive syndromic hearing impairment
| Disorder | Subtype | Gene |
|---|---|---|
| Usher syndrome | Type I | CDH23,MYO7A,PCDH15,USH1C,USH1G |
| Type II | USH2A,GPR98 | |
| Type III | CLRN1 | |
| Pendred syndrome | SLC26A4 | |
| Jervell and Lange-Nielsensyndrome | KCNE1,KCNQ1 | |
| Refsum disease | PHYH,PEX7 |
X-linked syndromic hearing impairment
| Disorder | Subtype | Gene |
|---|---|---|
| Alport syndrome | COL4A3,COL4A4,COL4A5 | |
| Mohr-Tranebjaerg syndrome | TIMM8A |
Mitochondrial syndromic hearing impairment
| Disorder | Subtype | Gene |
|---|---|---|
| Kearns-Sayre syndrome | MELAS,MERRF,NARP |
Non-Syndromic Hearing Impairment
More than 70% of hereditary hearing loss is non-syndromic. The different gene loci for nonsyndromic deafness are designated DFN. Loci are named based on mode of inheritance.
- DFNA: Autosomal dominant
- DFNB: Autosomal recessive
- DFNX:X-linked
Most autosomal dominant loci cause post-lingual hearing impairment. There is no identifiable single gene responsible for majority of cases (Table-1).
Table 1- Known genes causing autosomal dominant non-syndromic hearing impairment| Locus Name | Gene | Onset/Decade | Audio profile |
|---|---|---|---|
| DFNA1 | DIAPH1 | Postlingual/1st | Low frequency progressive |
| DFNA2 | KCNQ4 | Postlingual/2nd | High frequency progressive |
| DFNA2B | GJB3 | Post lingual/4th | High frequency progressive |
| DFNA3 | GJB2, GBJ6 | Prelingual | High frequency progressive |
| DFNA4 | MYH14 | Postlingual | Flat/gently downsloping |
| DFNA5 | DFNA5 | Postilngual/1st | High frequency progressive |
| DFNA6/14/38 | WFSI | Prelingual | Low frequency progressive |
| DFNA8/12 | TECTA | Prelingual | Mid-frequency loss |
| DFNA9 | COCH | Postlingual/2nd | High frequency progressive |
| DFNA10 | EYA4 | Post lingual/3rd,4th | Flat/gently down sloping |
| DFNA11 | MYO7A | Post lingual/1st | Flat/gently down sloping |
| DFNA13 | COL11A2 | Postlingual/2nd | Mid-frequency loss |
| DFNA15 | POU4F3 | Post lingual | High frequency progressive |
| DFNA17 | MYH9 | Post lingual | High frequency progressive |
| DFNA20/26 | ACTG1 | Post lingual | High frequency progressive |
| DFNA22 | MYO6 | Post lingual | High frequency progressive |
| DFNA23 | SIX1 | Prelingual | Down sloping |
| DFNA25 | SLC17A8 | Postlingual/2nd-6th decades | High frequency progressive |
| DFNA28 | GRHL2 | Postlingual | Flat/Gently downsloping |
| DFNA36 | TMC1 | Postlingual | Flat/Gently downsloping |
| DFNA39 | DSPP | Postlingual | High frequency progressive |
| DFNA41 | P2RX2 | Postlingual | Flat progressive |
| DFNA44 | CCD50 | Postlingual | Low to mild frequencies progressive |
| DFNA48 | MYO1A | Postlingual | Progressive |
| DFNA50 | MIR96 | Postlingual/2nd | Flat progressive |
| DFNA51 | TJP2 & FAM189A2 | Postlingual/4th | High frequency progressive |
Most autosomal recessive loci cause prelingual severe-to-profound hearing loss. An exception is DFNB8, in which the hearing impairment is prelingual and rapidly progressive. About 50% of autosomal recessive nonsyndromic hearing loss has been attributed to mutations in the gene GJB2. The remaining 50% have mutations in numerous other genes (Table-2).
Table 2- Known genes causing autosomal recessive non-syndromic hearing impairment| Locus Name | Gene | Onset | Type |
|---|---|---|---|
| DFNB1 | GJB2, GJB6 | Prelingual | Usually stable |
| DFNB2 | MYO7A | Prelingual, Postlingual | Unspecified |
| DFNB3 | MYO15A | Prelingual | Severe to profound; stable |
| DFNB6 | TMIE | Prelingual | Severe to profound; stable |
| DFFNB9N7/11 | TMC1 | Prelingual | Severe to profound; stable |
| DFNB8/10 | TMPRSS3 | Postlingual, Prelingual | Progressive, stable |
| DFNB9 | OTOF | Prelingual | Usually severe to profound;stable |
| DFNB12 | CDH23 | Prelingual | Severe to profound; stable |
| DFNB16 | STRC | Prelingual | Severe to profound; stable |
| DFNB18 | USH1C | Prelingual | Severe to profound; stable |
| DFNB21 | TECTA | Prelingual | Severe to profound; stable |
| DFNB22 | OTOA | Prelingual | Severe to profound; stable |
| DFNB23 | PCDH15 | Prelingual | Severe to profound; stable |
| DFNB24 | RDX | Prelingual | Severe to profound; stable |
| DFNB25 | GRXCR1 | Prelingual | Moderate to profound ; progressive |
| DFNB28 | TRIOBP | Prelingual | Severe to profound; stable |
| DFNB29 | CLDN14 | Prelingual | Severe to profound; stable |
| DFNB30 | MYO3A | Prelingual | Severe to profound; stable |
| DFNB31 | CHRN | Prelingual | - |
| DFNB32/82 | GPSM2 | Prelingual | Severe to profound; stable |
| DFNB35 | ESRRB | Unknown | Severe to profound |
| DFNB36 | ESPN | Prelingual | - |
| DFNB37 | MYO6 | Prelingual | - |
| DFNB39 | HGF | Prelingual | Severe to profound; down sloping |
| DFNB49 | MARVELD2 | Prelingual | Moderate to profound; stable |
| DFNB53 | COL11A2 | Prelingual | Severe to profound; stable |
| DFNB59 | DFNB59 | Prelingual | Severe to profound; stable |
| DFNB61 | SLC26A5 | Prelingual | Severe to profound; stable |
| DFNB63 | LRTOMT | Prelingual | Severe to profound; stable |
| DFNB67 | LHFPL5 | Prelingual | Severe to profound; stable |
| DFNB73 | BSND | Prelingual | Severe to profound; stable |
| DFNB76 | SYNE4 | Prelingual | High frequency; progressive |
| DFNB77 | LOXHD1 | Postlingual | Moderate to profound; progressive |
| DFNB79 | TPRN | Prelingual | Severe to profound; stable |
| DFNB84 | PTPRQ | Prelingual | Moderate to profound; progressive |
X-linked nonsyndromic hearing loss can be either pre-or postlingual. Mutations of PRPS1, POU3F4 and SMPX have been identified in X-linked syndromic hearing impairment (Table-3).
Table 3- X-Linked nonsyndromic hearing impairment causative genes| Locus Name | Gene | Onset | Type and Degree | Frequencies |
|---|---|---|---|---|
| DFNX1 (DFN2) | PRPS1 | Postlingual | Progressive sensorineural;severe to profound | All |
| DFNX2 (DFN3) | POU3F4 | Prelingual | Progressive,mixed;variable,but progresses to profound | All |
| DFNX4 (DFN6) | SMPX | Postlingual | Progressive sensorinueral;mild to profound | All |
Mutations in the mitochondrial genes MT-RNR1, MT-TS1, and/or MT-CO1 have been reported to cause non-syndromic hearing loss (Table-4).
Table 4- Mitochondrail genes responsible for non-syndromic hearing impairment| Gene | Mutation | Severity | Penetrance |
|---|---|---|---|
| MT-RNRI | 961 different mutations, 1494C>T, 1555A>G | Variable | Highly variable, Aminoglycoside induced |
| MT-TSI | 7445A>G, 7472insC, 7510T>C, 7511T | Variable | Highly variable |
| MT-CO1 | 7444G>A | Severe to profound | Complete, aminoglycoside associated ; associated with MT-RNR1 1555A>G |
Molecular Genetic Testing
Genetic testing for the cause of non-syndromic hearing impairment is available by combining the polymerase chain reaction and DNA sequencing techniques. Sequencing of GJB2 and GJB6 should be considered first, in the evaluation of individuals with congenital non syndromic sensorineural hearing loss. In other cases, the extreme genetic heterogeneity and frequent lack of phenotypic variability make genetic diagnosis difficult using single gene screening technique. Therefore multi-gene screening panels have been developed to screen for the cause of hearing loss by different laboratories and groups. These screening panels vary by laboratory both in the techniques used and number of genes sequenced.
